

CONTENTS
- Quality of Sewage
- Characteristics of Sewage
- BOD by Dilution Technique
- Ultimate First Stage BOD
- BOD/COD Ratio
- Sewage Disposal into Streams
- Sewage and Sewerage Treatment
- Sewer Materials
- Laying of Sewers
- Surface Drains
- Shapes of Surface Drains
- Sewer Appurtenances
- Sewage Treatment
- Sewage Treatment Process
- Design of Trickling Filters
- Activated Sludge Plant
- Sludge Volume Index (S.V.I.)
- Methods of Aeration, and Aerators
- Sludge Digestion Process
- Aerobic and Anaerobic Biological Units
- Design Criteria
- Disposal of Sewage Effluents
- Disposal of Solid Wastes and Refuse of a Society
- Disposal of Refuse
- Methods of Sludge disposal

QUALITY OF SEWAGE
Characteristics and Quality of sewage must be determined before its disposal because of following:
- Floating solids of untreated sewage decompose and create unpleasant smells and odour in the river water.
- Large amount of organic matter present in untreated sewage starts consuming dissolved oxygen of the river water. Due to less amount of dissolved oxygen in river water, fish start dying.
- Untreated sewage is also responsible for contaminating source water with harmful micro-organisms called pathogenic bacterias. Pathogens are responsible for causing serious water borne diseases such as cholera, typhoid, dysentry, etc.
Though municipal sewage normally contains 99.9% of water content, it is always desirable to treat the sewage before discharging the same in the river water to safe guard against the above defects. Factors deciding Extent and Type of treatment required for the Sewage so as not to pollute Source of disposal: 1. Character and quality of sewage 2. Source of disposal
Treatment of Sewage
Out of millions bacterias generally found per litre of untreated sewage, only a small number are harmful to man. These harmful bacterias are called pathogens. The remaining large number of bacterias called non-pathogens are not only harmless but useful for the process of decomposition of the sewage. The main basis of treatment of sewage is to provide a suitable environment for the action of aerobic and anaerobic bacterias for stabilising organic matter present in sewage either through aerobic or anaerobic decomposition.
IES MASTER CIVIL ENGINEERING GATE STUDY MATERIALS PDF: DOWNLOAD LINK
ACE ACADEMY CIVIL ENGINEERING GATE STUDY MATERIALS PDF: DOWNLOAD LINK
Decomposition of Sewage
- Aerobic decomposition. During treatment, in aeration tanks, contact beds, intermittent sand filters, trickling filters and oxidation ponds, it is primarily done by oxidation.
- Anaerobic decomposition. During treatment, in septic tanks, Imhoff tanks and sludge digestion tanks, it is mainly done by putrefaction alone.
Physical Characteristics of Sewage
- Turbidity – Degree of turbidity of sewage may be measured either by a turbidity rod or turbid meter. The degree of turbidity increases with the increase of sewage strength.
- Colour – Colour of sewage indicates degree of its freshness. Black or dark brown colours indicate stale and septic sewage.
- Odour – Fresh sewage remains practically odourless. As soon as dissolved oxygen gets exhausted, the sewage first becomes septic and thereafter offensive odours are evolved due to decomposition of sewage. Hydrogen sulphide gas is generally liberated from stale decomposed sewage.
- Temperature – Temperature of untreated sewage affects following
- Biological activities of the bacterias present in the sewage.
- Solubility of gases in the sewage.
- Viscosity of sewage which ultimately affects the sedimentation process.
Average temperature of sewage in India is about 20C which is favourable for the biological activities. At higher temperature, dissolved oxygen gets reduced considerably. 5. Soli ds. It contain 99.9% water and 0.1% solids.
- Solids – It contain 99.9% water and 0.1% solids.
Chemical Characteristics of Sewage
Total Solids – Solids may exist in the sewage in any of the following forms:
- Suspended solids: These solids remain floating in sewage.
- Dissolved solids: These solids remain dissolved in sewage.
- Colloidal solids: These are finely divided solids which remain either in solution or in suspension.
- Settleable solids: These are solid matter which settles at the bottom of the container in case sewage-is kept undisturbed for a period of two hours.


pH value
Logarithm of reciprocal of hydrogen ion concentration present in sewage, is called pH value. If pH value is less than 7, the sewage is acidic, and if more than 7, the sewage is alkaline. pH value may be determined with the help of a potentiometer.
Chloride Contents
Chloride upto 120 mg/ litre is obtained from domestic sewage. Large quantity of chlorides is added from industrial waste. High content of chloride in the sewage indicates presence of industrial waste. Chloride content in the given sample of sewage may be measured by titrating with standard silver nitrate solution, using potassium chromate as indicator. Two tests are conducted for chlorine
- Chlorine Demand Test: This test is done to determine amount of chlorine required for proper disinfection. Unstable organic matter present in sewage has a demand for chlorine and the amount of chlorine required for this purpose is called chlorine demand. It thus indicates the amount of organic matter present in the sewage.
- Chlorine Residual Test: After treatment of sewage, it is necessary to chlorinate it to kill any bacteria present. If residual chlorine is present after its application, it indicates that chlorination is sufficient. Residual chlorine test is conducted in the same manner as that for water.
ACE ACADEMY CIVIL ENGINEERING GATE HANDWRITTEN CLASSROOM NOTES PDF: DOWNLOAD LINK
MADE EASY CIVIL ENGINEERING GATE HANDWRITTEN CLASSROOM NOTES PDF: DOWNLOAD LINK
Nitrogen contents
Presence of nitrogen in sewage indicates presence of organic matter. It may occur in one or more of the following forms
- Free ammonia: During first stage of decomposition of organic matter, free ammonia is liberated. The amount of free ammonia present in sewage is measured by simply boiling sewage, and measuring the gas thus liberated.
- Albuminoid nitrogen: Quality of nitrogen present in sewage before commencement of decomposition of organic matter indicates the albuminoid nitrogen. The amount of albuminoid nitrogen may be measured by adding strong alkaline solution Pottasium Permaganate (KMnO4) to the already boiled sewage sample and again boiling the same. Ammonia gas thus liberated is required quantity of albuminoid nitrogen in the given sample.
- Nitrites: Presence of nitrites indicates that organic matter in the sewage in only partly decomposed. Quantity of nitrites present in the sewage sample may be measured by colour matching method by adding sulphonorilic acid and naphthamine. The colour developed in the water is compared with standard colour of solution of known concentration.
- Nitrates: Presence of nitrates in the sewage indicates that organic matter is fully oxidised. Amount of nitrates present in the sewage sample may be measured by colour matching method by adding phenol-di-sulphuric acid and potassium hydroxide. Colour developed in the waste water is compared with standard colour of known concentration
- Presence of Fats and Grease: Sources of grease, fats, and oils in the sewage is from the discharges of animal and vegetable matter. These matters forms scum on the top of the sedimentation tanks, and clog voids of the filtering media. To determine amount of fats and grease, sewage sample is first evaporated and the residual solids so left are mixed with either ether or hexane. Thus solution obtained is allowed to evaporate. The residue is of fats and grease.
- Hydrogen Sulphide gas: Presence of hydrogen sulphide gas in sewage indicates anaerobic decomposition. Excess amount of hydrogen sulphide gas may cause corrosion of concrete sewers and may produce bad odours at the treatment plant. To safeguard against these bad effects, hydrogen sulphide gas (H2S) is kept below 1 ppm in fresh sewage.
- Dissolved oxygen (DO): Because of rapid absorption of oxygen from the atmosphere, dissolved oxygen is always present in variable quantities in sewage water. Its content in sewage is dependant upon amount and character of unstable organic matter in it. The test of dissolved oxygen is carried out before discharging treated sewage into source water to ensure that at least 4 ppm of DO is available in the sewage for the existence of fish life. DO content of sewage is generally determined by Wrinkler’s method which depends on the fact that, in alkaline solution, the dissolved oxygen oxidises magnaneous ion to maganic ion which in turn oxidises iodide to liberate iodine in quantities equivalent to the amount of dissolved oxygen present. The dissolved oxygen is reported as mg/l or as percentage saturation with dissolved oxygen.
Present of fats, greases, oils, sulphides, sulphates and H2S gases.
Strength of Sewage
It gives an indication of the nuisance value of sewage. It is generally indicated by following characteristics:
- Total volatile solids, both suspended and dissolved
- Odour
- Chlorine demand
- Theoretical Oxygen Demand (T.O.D). Amount of oxygen required for complete oxidation of organic matter into CO2 is called TOD.
Chemical Oxygen Demand (COD)
Amount of oxygen required for chemical oxidation is called COD. It is defined as amount of oxygen absorbed by waste water from a strong oxidising agent like K2Cr2O7, KmnO4. Importance of Chemical Oxygen Demand is due to following reasons:
- Rapid chemical oxidation.
- Chemical oxidation does not depend on many variables.
- Chemical oxidation requires less equipment, hence economical.
- In highly toxic sewage, chemical procedure is the only method lo determine the organic load. Method of determination of COD: It is Refluxing. COD results although less than T.O.D and depends on composition. Time required for COD test is 3 to 4 hrs.
Advantages of COD test
- Computation of various parameters are not required
- Time required for conducting COD test is less than T.O.D. test
Disadvantages of COD test – This does not differentiate between biodegradable organic matter and nonbiodegradable organic matter.
Total Organic Carbon (TOC)
This test involves oxidation of the sample to convert inorganic carbon to CO2, which is then stripped. Both COD and TOC measures biodegradable fraction of the organics, but unlike COD it is independent of the oxidation state of the organic matter. CO2 released in the test can be measured by a infrared analyser. This test is rapid, accurate and correlates moderately well with BOD.
Biochemical Oxygen Demand (BOD)
BOD is most commonly used parameter to define strength of municipal or organic industrial waste water. It is defined as the amount of oxygen required by micro-organisms for the decomposition of bio-degradable matters under aerobic condition. Standard BOD test: It determines the amount of oxygen required by micro-organism for decomposition of bio-degradable matter under aerobic condition in 5 days at 200C.
ENVIRONMENTAL ENGINEERING ACE ACADEMY GATE CLASSROOM NOTES PDF: DOWNLOAD LINK
ENVIRONMENTAL ENGINEERING MADE EASY GATE CLASSROOM NOTES PDF: DOWNLOAD LINK
BOD by Dilution Technique
BOD tests consists of diluting the sewage with water containing a known amount of dissolved oxygen and noting the loss of oxygen after a period of storage or incubation. The usual incubating period is 5 days at a temperature of 20C. The diluting water is aerated, contains a small amount of sodium bicarbonate and has a pH of 7.0 to 7.6. The rate of biochemical oxidation of organic matter is proportional to remaining concentration of the unoxidized substance that is measured in terms of oxidizability. The relationship is shown graphically.
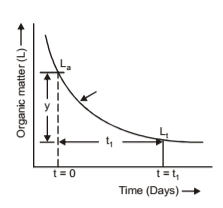
From the figure, L is the oxygen demand at t = 0 day. It may also be called first stage demand though the same relationship applies at the beginning of oxidation period. Then Lt is remaining oxygen demand at the end of any time ‘t’ days. K is a constant associated with days and temperature and determinable experimentally.
Biological Characteristics of Sewage
Sewage contains living organisms, such as bacteria, algae, fungi and protozoa. Following two types of bacteria in sewage carry out the process of breaking the complex organic compounds into simple and stable compounds:
- Aerobic bacteria: It live in the presence of oxygen dissolved in water or free oxygen.
- Anaerobic bacteria: It live and carry on their activities in the absence of free oxygen.
Decomposition of sewage
It takes place in following two stages:
- Aerobic Decomposition – Sewage contains organic matter, waster products, water, etc. Aerobic bacteria convert this matter, in the presence of the dissolved oxygen in the sewage water, initially to nitrogeneous, carbonaceous and sulphurous compounds, which are more stable. With the supply of more oxygen, these compounds are further decomposed into more stable nitrites and then to nitrates. Aerobic decomposition is also called oxidation, because during this process, organic matter is broken up and oxidised to more stable products. Aerobic bacteria produce gases which are not offensive in odour. When oxygen supply in the water is exhausted, the aerobic bacteria die. Following treatment plants work on the oxidation principle: Aeration tanks, Contact beds, Intermittent sand filters, Trickling filters and Oxidation ponds.
- Anaerobic Decomposition – When aerobic bacteria die, anaerobic bacteria start their activity with the oxygen available in the organic matter. These bacteria break up organic compounds to nitrites, nitrates, proteins, etc. The gases produced in the process are very offensive in odour. Anaerobic decomposition is also called putrefaction and the end products include black residue called humus, ammonia, methane, hydrogen sulphides, etc. Following treatment units work on the principle of putrefaction: Septic tanks, Imhoff tanks, Sludge digestion tanks, etc. Plants use products of decomposition such as carbon dioxide or nitrates to produce chlorophyll. When plants die they are decomposed by aerobic and anaerobic bacteria and so the cycle goes on.
Fresh sewage does not have offensive odour. But after a few hours it becomes stale, septic and foul. Hence in sewage treatment, aerobic decomposition is encouraged by supplying oxygen for its activity by the following ways:
- Allowing sewage to pass through porous medium, and circulating through the pores as in the case of trickling filters.
- Adding activated sludge to fresh sewage and blowing air. In a sewage treatment plant, activity of anaerobic bacteria is controlled so that odour is not noticeable.
SEWAGE AND SEWERAGE TREATM ENT
Types of Sanitary Sewage
- Domestic sewage
- Industrial sewage
- Storm sewage
System of Sanitation
- Old conservancy system
- Modern water carriage system
Types of Sewerage System
- Combined system
- Separate system
- Partially separate system
Components of Sew age System
- House sewers
- Lateral sewers
- Branch sewers
- Main sewers (Trunk sewers)
- Outfall sewers
- Manholes
Infiltration
It is the water that enters sewers through poor joints, cracks, manhole covers, etc. During dry weather, there will be no infiltration and hence only domestic sewage and industrial waste will be conveyed. During rains, infiltration will be due to rise in ground water table and from roofs. Infiltration depends on the following factors:
- Height of ground water level
- Type of soil in which sewers are laid
- Workmanship of laying pipes
Dry Weather Flow
Minimum sewage discharge through combined sewerage system during non-monsoon period is called dry weather flow (D.W.F). Drainage discharge, which is produced during monsoon season is generally very high, say 20 to 25 times that of the sewage discharge called dry weather flow (D.W.F). Quantity of dry weather flow depends on following factors
- Population: As in a water-supply project, probable life of the sewage system has to be fixed according to the life of different components, say 40 or 50 years. The population to be served at the end of the period will have to be determined to fix size of sewers and other components of the system.
- Rate of Water Supplied: Quantity of domestic sewage entering the sewer depend on the water-supply. However, all the water supplied may not reach the sewer as part of it may have been used for a purpose such as watering gardens, which may not return to the sewer. It may also happen that industries may have their own supply of water which may be led into sewers. Thus it is usually assumed that average rate of sewage flow equals average rate of consumption of water. Thus following two factors should be closely checked before deciding proportion of water-supply appearing as sewage: (a) Purpose for which water is being used has to be carefully studied. (b) Intensity of pressure in the pipelines has to be checked. More the pressure, more the wastage of water and leakage and so quantity reaching consumers will be less than that supplied.
- Nature of Industries: Quantity of industrial waste depends on the type of industry. Hence, each industry has to be carefully studied before estimating quantity of industrial sewage.
SOLID WASTE AND WASTEWATER TREATMENT CIVIL ENGINEERING GATE 2020 STUDY MATERIAL FREE DOWNLOAD PDF
DOWNLOAD LINK : CLICK HERE
PASSWORD : CivilEnggForAll
OTHER USEFUL BOOKS
- CIVIL ENGINEERING TEXTBOOKS WITH DOWNLOAD LINKS
- IES MASTER CIVIL ENGINEERING GATE STUDY MATERIALS PDF
- ACE ACADEMY CIVIL ENGINEERING GATE STUDY MATERIALS PDF
- BUILDING MATERIALS – MOCK TEST 1 (QUICK)
- TELANGANA STATE PUBLIC SERVICE COMMISSION – ASSISTANT ENGINEER 2023 – TSPSC AE 2023 CIVIL ENGINEERING EXAM SOLVED PAPER WITH EXPLANATIONS PDF FREE DOWNLOAD
- SSC JE 2023 CIVIL ENGINEERING (CPWD/CWC/MES) EXAM SOLVED PAPER PDF FREE DOWNLOAD
- BIHAR PUBLIC SERVICE COMMISSION ASSISTANT ENGINEER (BPSC AE) 2022 CIVIL ENGINEERING EXAM SOLVED PAPER WITH EXPLANATIONS PDF
- NHPC (NATIONAL HYDROELECTIC POWER CORPORATION) JUNIOR ENGINEER NHPC JE 2022 CIVIL ENGINEERING EXAM SOLVED PAPER PDF FREE DOWNLOAD

Leave a Reply