
CONTENTS
- Classification of Air Pollution
- Sources of Air Pollution
- Effects of Air Pollution
- Air Pollution Meteorology
- Inversions
- Wind Velocity Profiles
- Stack Plumes
- Air Pollution Control
- Composition of Clean and Dry Atmospheric Air

Air pollution is defined as ‘‘presence in the outdoor atmosphere of one or more contaminants, such as dust, fumes, gas, mist, odour, smoke or vapour, in quantities, of characteristics, and of duration such as injurious to human, plant or animal life or to property, or which unreasonably interferes with comfortable enjoyment of life and property’’.
- Aerosol: It is a dispersion of solid or liquid particles of microscopic size in gaseous media, such as smoke, fog or mist.
- Dust: It is a loose term applied to solid particles larger than colloidal particles and capable of temporary suspension in air or other gases. Dusts do not tend to flocculate except under electrostatic forces; they do not diffuse but settle under the influence of gravity. (Size: 1 – 200 μm)
- Droplet: It is a small liquid particle of such size and density as to fall under still conditions but which may remain suspended under turbulent conditions.
- Fly-ash: It is finely divided particles of ash entrained in fuel gases arising from the combustion of fuel. The term is generally applied to the gas-borne ash from boilers using pulverised fuel (coal) firing.
- Fog: It is a loose term applied to liquid dispersed aerosols in air by condensation.
- Fume: It is solid particles generated by condensation from the gaseous state, generally after volatilization from melted substances, and often accompanied by a chemical reaction such as oxidation. (Size: 0.1 to 1μm)
- Mist: It is a loose term applied to dispersions of liquid particles in atmosphere, the dispersion large size. (Size: 5– 100μm)
- Particle: It is a small discrete mass of solid or liquid matter.
- Smoke: It is finely divided aerosol particles resulting from incomplete combustion. It consists mainly of carbon and other combustible material. (Size: 0.01 to 1μm)
- Soot: It is collection of particles of carbon impregnated with ‘tar’ formed in the incomplete combustion of carbonaceous material.
- Vapour: It is the gaseous form of matter which normally exists in a liquid or solid state.
IES MASTER CIVIL ENGINEERING GATE STUDY MATERIALS PDF: DOWNLOAD LINK
ACE ACADEMY CIVIL ENGINEERING GATE STUDY MATERIALS PDF: DOWNLOAD LINK
CLASSIFICATION OF AIR POLLUTION
Air pollution exists in three distinct categories
- Personal air pollution – This refers to exposure of an individual to dust, fumes and gases. e.g. person indulges in cigarette, cigar or pipe smoking.
- Occupational air pollution – This represents the type of exposure of individuals to potentially harmful concentration of aerosols, vapours and gases in their working environment.
- Community air pollution – This represents most complex of the three varieties since it involves pollution from a variety of sources and contaminants and factors which cause adverse social, economic and health effects. Not only does community air pollution affect many individuals, but it can also exert a significant impact on man’s total environment including plants, animals, property and the weather itself.
SOURCES OF AIR POLLUTION
There are two main sources of air pollution
1. Natural air pollution sources
Atmosphere is polluted due to following natural causes:
- Wind-blown dust
- Smoke, fly-ash, gases from forest fires
- Micro-organisms
- Gases and odours from swamps and marshes
- Fog
- Volcanic ash and gases
Pollutants from various natural sources
- Pollutant natural sources
- SO2 Volcanoes
- H2S Volcanoes, biological action in swamp areas
- CO Forest fires, ocean
- NO-NO2 Bacterial action in soil
- NH3 Biological decay
- Hydrocarbons
- (CH4, etc.) Biological processes
- CO2 Biological decay, release from oceans
- O3 Oxygen and ozone in stratosphere and their downward transport
2. Man-Made Air Pollution sources
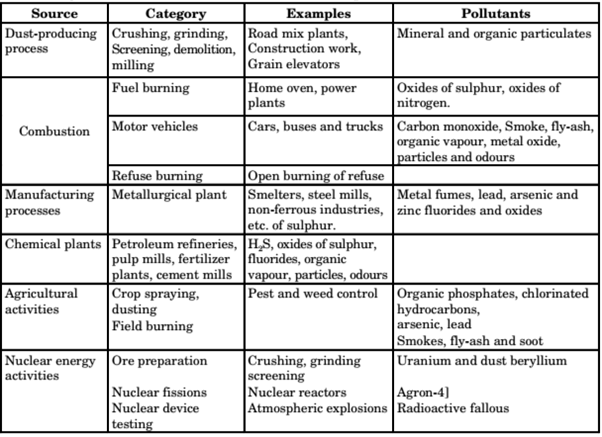
EFFECTS OF AIR POLLUTION
1. Effect on Vegetation – Vegetation exposed to atmosphere containing heavy amounts of sulphur dioxide is severely damaged. It is also affected by ozone and nitrogen dioxide.
2. Effect on Human beings and Animals – People suffer from respiratory diseases when exposed to atmosphere in which concentration of the sulphur dioxide is in excess of safe limits, and their visibility is affected when exposed to higher temperature. The death rate of people suffering with cardiac or pulmonary diseases, when exposed to atmosphere contaminated with sulphur dioxide, is found to be higher. Due to incomplete combustion of fuels from petrol engines, industrial operations, etc., carbon monoxide is liberated.
When air containing CO is inhaled, CO combines with the haemoglobin of the blood, depriving the tissues of oxygen. It has been found that when carbonyl haemoglobin saturation level of blood is about 20%, it harms heart and also impairs tissues restricting the oxygen. When in excess of 10%, it is found to cause headaches. When nitrogen dioxide level in the air is above tolerable limits, respiratory illness among children has been observed. When oxygen content in the air is above 500 mg/m3 and photochemical action is high, people have been found to have asthmatic attacks. Irritation of throat, nose or eyes are some other minor problems experienced in these conditions. In general, air pollution decreases visibility. It is also found that it makes the town or city more cloudy, more foggy and is subjected to more acid rains.
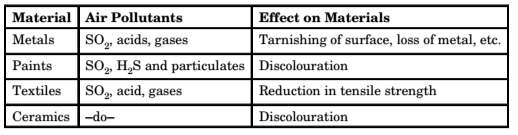
3. Effect on Materials – Air pollutants affect materials in the following ways and cause economic losses
- Abrasion
- Deposition of materials
- Direct chemical attack
- Indirect chemical attack
- Corrosion
ACE ACADEMY CIVIL ENGINEERING GATE HANDWRITTEN CLASSROOM NOTES PDF: DOWNLOAD LINK
MADE EASY CIVIL ENGINEERING GATE HANDWRITTEN CLASSROOM NOTES PDF: DOWNLOAD LINK
AIR POLLUTION METEOROLOGY
Atmospheric conditions can have profound effects on pollution and its harmfulness, and air pollution meteorology is concerned with the description of the transport of pollutants from sources to receptors. Pollutants emitted into the atmosphere are subject to following four types of effects:
- Transport
- Dilution
- Modification
- Removal
Temperature and Pressure Relationship in the Lower Atmosphere
Change of temperature with altitude has great influence on the motion of air pollutants. e.g., very stable atmospheric conditions result in only limited vertical mixing.
Stability Conditions
Value of the lapse rate in the lower portion of the troposphere has a profound influence on the vertical motion of the air. Good vertical mixing minimizes immediate ground-level effects of air pollutants, since contaminants may be quickly diluted through their dispersal into higher regions. If air does not vigorously mix upwards, pollutants which are released at low levels tend to remain there. Stable atmosphere is defined as the one which does not exhibit much vertical mixing or motion. Degree of mixing is primarily dependent upon following
- Temperature gradient
- Mechanical turbulence
The possibility of thermal mixing can be determined by comparison of actual (environmental) temperature gradient or lapse rate to adiabatic lapse rate. Internal energy decreases thereby decreasing temperature. Upon reaching new height, if temperature of the air particle is same as the temperature of the environment, then environmental lapse rate is exactly same as adiabatic lapse rate. In other words, air particle will have same pressure, temperature and density of the surroundings and would see no buoyant force. Such type of atmosphere with an adiabatic lapse rate is called neutrally stable.
In this, a displaced mass of air neither tends to return to its original position nor tends to continue its displacement. Now consider that atmosphere temperature decreases less rapidly than the adiabatic lapse rate. Air particle follows a temperature change given by the adiabatic slope, but when it arrives at new height it is at a lower temperature than the surrounding air whose temperature lies along the environmental temperature gradient line. This small parcel of air is thus more dense (since it is at the same pressure) than the surroundings and tends to fall back to its original position. Such an atmospheric condition is called stable .Under these conditions there is very little movement of air from one altitude to another so any pollutants will only slowly disperse. With no vertical mixing, pollution concentrations can build very rapidly. This stable atmospheric condition is also called sub-adiabatic condition where environmental lapse rate is less than dry adiabatic lapse rate. Thus any sub-adiabatic atmosphere is stable. The extreme case, called ‘Inversion’ occurs when temperature increases with altitude, forming a very stable atmosphere.
When environmental lapse rate is greater than the dry adiabatic lapse rate, then atmosphere is called super-adiabatic (i.e., actual temperature gradient is more negative than the dry adiabatic temperature gradient). Super-adiabatic atmosphere is unstable. Here air from one altitude eagerly mixes with the air from other altitudes. This is very desirable from the point of pollution, since pollutants will be rapidly dispersed throughout the atmosphere.
ENVIRONMENTAL ENGINEERING ACE ACADEMY GATE CLASSROOM NOTES PDF: DOWNLOAD LINK
ENVIRONMENTAL ENGINEERING MADE EASY GATE CLASSROOM NOTES PDF: DOWNLOAD LINK
INVERSIONS
Inversion layers play an important role in influencing dispersion of air pollutants by restricting vertical mixing, can be formed in many ways.
Subsidence Inversion
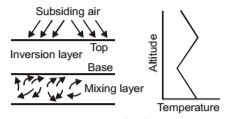
In this air is warmed by compression as it descends in a high pressure system such as a subtropical anticyclone. As it descends, air achieves a temperature higher than that of the air underneath. Thus subsiding air establishes a temperature inversion with respect to the low-laying air. This condition and temperature profile for a so-called elevated subsidence inversion. Subsidence is caused by air flowing down to replace air which has flown out of the high pressure region
Radiational Inversion
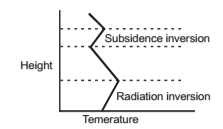
Height Subsidence inversion Radiation inversion Temperature It occurs wherever, surface of the earth can become cooler during the night by the thermal radiation of energy. As a result of a decrease in temperature of the ground, lower atmosphere in contact with it loses sensible heat through conduction, conversion, and radiation. Consequently a temperature inversion is setup between cool low-level air and the warmer air above. This type of inversion is most pronounced during late night are evident early in and early morning hours and is sometimes called nocturnal inversion. Effects of stable low-level air the day when smoke from chimneys and small fires is confined close to the ground; or if a wind is present, the smoke may form a pattern of narrow horizontal streaks as it disappears downwind. Nocturnal inversions are possible because surface of the earth can cool quickly. As the Sun warms earth during the day, inversion disappears. Pollutants may accumulate during the night beneath a very stable atmospheric level a few hundred meters high and then suddenly carried down to the ground in the morning when surface begins to warm and thermal convection leads to mixing of the air. This sudden increase in low-level pollution in the morning is called fumigation. Radiation inversion can occur near surface of the earth at the same time that a subsidence inversion exists at a higher altitude.
Advective Inversion
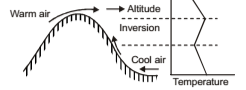
It occurs when warm air moves over a cold surface. Convective cooling of the lowest layer of air then leads to formation of a ground-based inversion. It also occurs when warm air is forced to move over top of a cooler layer. Here a hill range forces a warm land breeze to flow only at high levels, whereas a cool sea flows at low levels in the opposite direction. Note: Although an inversion is stable against air motion in the vertical direction, it does not discourage horizontal motion. Strong winds may be encountered within an inversion.
Classification of Stack Plumes
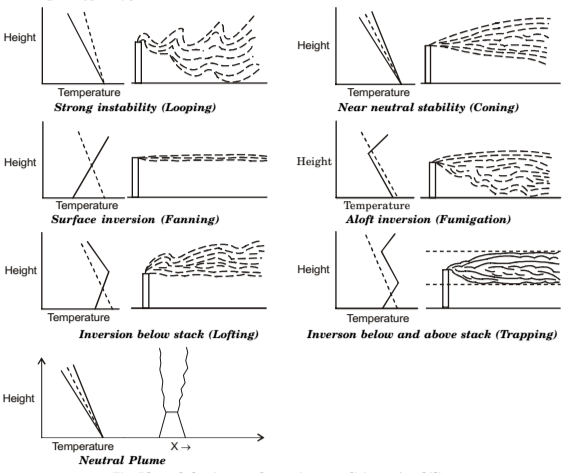
- Looping Plume. It occurs during unstable condition of a light wind on a hot summer afternoon, when large-scale thermal eddies carry portions of the plume to the ground level for short time periods. Plume touching the ground will yield a high pollutant concentration during that period. Looping plume indicates a super adiabatic lapse rate in the atmosphere.
- Coning Plume. It occurs when plume is dispersed by the wind under essentially neutral atmospheric stability. Since thermal heating effect is much lower than in the case of looping plumes, coning occurs under cloudy skies either the day or night. Here major part of the pollutant concentration is carried fairly far downwind before reaching ground level in significant amounts.
- Fanning Plume. It occurs when plume is dispersed under strong atmospheric inversions, during the evening, night or early morning. Atmosphere is extremely stable, and mechanical turbulence is suppressed. If density of the plume is not significantly different from that of the surrounding atmosphere, the plume travels downwind at approximately constant elevation. Little pollutant effluent reaches the ground.
- Fumigation Plume. It occurs when a stable layer of air lies a short distance above the release point of the plume and an unstable air layer lies below the plume. This condition usually arises when an inversion is breaking up in early morning when the sun comes up, i.e., when turbulent layer rising from the heated ground reaches a fanning plume (which has been emitted into and trapped at effective stack height in the inversion the night before), large concentrations of stack gas will be carried downwind to the surface. Fumigation is favoured by clear skies and light winds, and is more prevalent in the summer.
- Die lofting Plume. Conditions for these are inverse of these for the fumigation plume. It occurs when stack exhausts above an inversion, or when the plume buoyancy carries a stack emission through an inversion layer into an unstable layer above. Plume disperses above the inversion, since top of the inversion layer acts as a barrier that prevents all gaseous and small particle emissions from reaching the ground. This type of plume is major goals of tall-stack operation of electric-utility and industrial plants.
- Trapped Plume. It occurs when pollutant is emitted into an unstable layer of air trapped between inversions both below and above stack height. Diffusion of pollutants is severely restricted to the layer between two stable regions.
AIR POLLUTION CIVIL ENGINEERING GATE 2020 STUDY MATERIAL FREE DOWNLOAD PDF
DOWNLOAD LINK : CLICK HERE
PASSWORD : CivilEnggForAll
OTHER USEFUL BOOKS
- CIVIL ENGINEERING TEXTBOOKS WITH DOWNLOAD LINKS
- IES MASTER CIVIL ENGINEERING GATE STUDY MATERIALS PDF
- ACE ACADEMY CIVIL ENGINEERING GATE STUDY MATERIALS PDF
- RAJASTHAN STAFF SELECTION BOARD (RSSB) JUNIOR ENGINEER DIPLOMA CIVIL ENGINEERING EXAM 2022 – HINDI & ENGLISH MEDIUM SOLVED PAPER – FREE DOWNLOAD PDF (CivilEnggForAll.com)
- ISRO TECHNICAL ASSISTANT EXAM 2022 – CIVIL ENGINEERING – HINDI & ENGLISH MEDIUM – SOLVED PAPER – FREE DOWNLOAD PDF (CivilEnggForAll.com)
- MADHYA PRADESH PUBLIC SERVICE (MPPSC) COMMISSION – ASSISTANT ENGINEER EXAM – MPPSC AE 2021 CIVIL ENGINEERING – SOLVED PAPER WITH EXPLANATIONS – PDF FREE DOWNLOAD
- BIHAR PUBLIC SERVICE COMMISSION (BPSC) ASSISTANT ENGINEER EXAM – 2022 – CIVIL ENGINEERING – SOLVED PAPER – FREE DOWNLOAD PDF (CivilEnggForAll.com)
- ODISHA PUBLIC SERVICE COMMISSION – OPSC AEE PANCHAYATI RAJ EXAM 2021 – SOLVED PAPER WITH EXPLANATION – FREE DOWNLOAD PDF





















