
CONTENTS
- Water Demand
- Fire Demand
- Per Capita Demand
- Water Supply Scheme
- Design Periods & Population Forecasts
- Sources of Water
- Precipitations
- Rainfall And It’s Distribution
- Estimating Run-off And Yield of A Basin.
- Surface Sources Of Water Supplies
- Subsurface or Underground Water Sources
- Development of Ground Water
- Aquifers
- Groundwater Specific Yield
- Intake For Collecting Surface Water
- Conduits For Transporting Water
- Flows In Pipe Systems
- Analysis of Complex Pipe Networks
- Forces Acting On Pressure Conduits
- Pumps For Lifting Water
- Quality Control of Municipal And Industrial Water Supplies
- Chemical Characteristics
- Bacterial And Microscopical Characteristics
- Water Quality Standards For Drinking Water
- Biochemical Oxygen Demand
- Purification of Water Supplies
- Plain Sedimentation
- Design of Continuous Flow Type Of Sedimentation Tank
- Sedimentation Aided With Coagulation
- Mixing Basins
- Filtration
- Slow Sand Filters
- Disinfection Or Sterilisation
- Chlorination
- Water Softening
- Alkalinity
- Miscellaneous Treatments
- Removal Of Colours, Odours And Tastes From Water
- Removal of Iron And Manganese From Water
- Methods Of Distribution
- Quality Of Sewage
- Characteristics Of Sewage
- BOD By Dilution Technique
- Sewage Disposal Into Streams
- Temperature Dependence Of Rate Constant
- Sewage And Sewerage Treatment
- Sewer Appurtenances
- Sewage Treatment
- Sewage Treatment Process
- Aerobic And Anaerobic Biological Units
- Methods Of Sludge Disposal
- Noise Pollution
- Levels Of Noise
- Averaging Sound Pressure Levels
- Sources Of Noise
- Noise Abatement And Control

Water is extremely useful to man, providing him luxuries and concerts, in addition to fulfilling his basic necessities of life. It has been estimated that two third of human body is constituted of water. Suitable systems should be designed for collecting, transporting, and treating water.
Essential elements of a public water supply scheme:
- Intake and reservoir: To collect water.
- Water treatment plant: Screening, sedimentations, filtration, disinfection units etc.
- Elevated tanks and stand pipes: It provide storage to meet peak demands occurring for limited periods.
- Valves: It control the flow of water in the pipe system.
- Hydrants: It provide a connection with the water in the main for fighting fires, flushing streets etc.
- Distribution system: Mains, submains, and branch lines which carry the water to the streets.
- Services: It carry the water to the individual house etc.
WATER DEMAND
- In fact the first requirement is to consider the demand, and the second requirement is to find sources to fulfil that demand.
- Various Types of Water Demands:
- Domestic water demand – 55 to 60% of total water consumption.
- Industrial water demand – 50 mp
- Institutional and commercial water demand – 20 lpcd
- Demand for public uses – 10 lpcd
- Fire demand; –1 lpcd and
- Water required to compensate losses in wastes and thefts – 55 lpcd
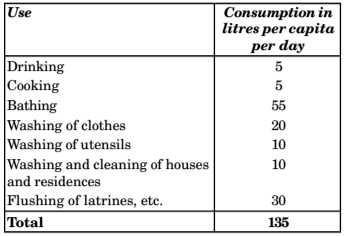
- As per is 1172-1983 as well as National building code, the domestic consumption under normal conditions in an Indian city is expected to be around 135 litre/ head/day.
- The ordinary per capital consumption for industrial needs of a city is generally taken as 50 litres/capita/day
- On an average, per capita demand of 20 l/h/d is usually considered to be enough to meet such commercial and institutional water requirements, although this demand may be as high as 50 l/h/d for highly commercialised cities.
- Demand for public uses is generally taken as 10 l/h/day.
ENVIRONMENTAL ENGINEERING IES MASTER GATE STUDY MATERIAL PDF: CLICK HERE
PRECIPITATION
Types of Precipitations
- Cyclonic precipitation;
- Convective precipitation; and
- Orographic precipitation.
• The usual mechanism by which the air is cooled to cause precipitation is the lifting of the air mass.
- Cyclonic precipitation
- It is caused by the lifting of the air mass due to the pressure difference. If low pressure occurs in an area, air will flow horizontal from the surrounding area, causing the air in the low pressure area to lift. The precipitation that results, is called non-frontal cyclonic precipitation. If one air mass lifts over another air mass, then the precipitation is called frontal or cyclonic precipitation.
- The boundary between these two air masses of different temperatures and densities is known as a front or a front al sur face.
- The large whirling mass of air, at the centre of which the barometric pressure is low, is known as a cyclone.
- Convective precipitation: Convective precipitation is due to the upward movement of air that is warmer than its surroundings. Precipitation occurs of high intensity and short duration.
- Orographic precipitation: Orographic precipitation is the most important precipitate which is responsible for most of the heavy rains in India. Orographic precipitation caused by air masses which strike some natural topographic barriers like mountain and cannot move forward, and hence rise up, causing condensation and precipitation.
Hardness of water:
- Hard waters are undesirable because they may lead to greater soap consumption, scaling of boilers, causing corrosion and incrustation of pipes, making food tasteless etc.
- If bicarbonates and carbonates of calcium and magnesium are present in water, the water is render hard temporarily as this hardness can be removed to some extent by simple boiling or to full extent by adding lime to water. Such a hardness is known as temporary hardness or carbonated hardness.
- If sulphates, chlorides and nitrates of calcium or magnesium are present in water, they cannot be removed at all by simple boiling and therefore, such water require special treatment for softening. Such a hardness is known as permanent hardness or n on carbonate hardness. It is caused by Sulphates, Chlorides, Nitrates of Ca and Mg.
ENVIRONMENTAL ENGINEERING ACE ACADEMY GATE STUDY MATERIAL PDF: CLICK HERE
Turbidity
- The turbidity is measured by a turbidity rod or by a turbidimeter with optical observations, and is expressed as the amount of suspended matter in mg/lit or parts per million (ppm).
- For water, ppm and mg/lit are approximately equal.
- The standard unit is that which is produced by one milligram of finely divided silica (Fuller’s earth) in one litre of distilled water.
Turbidimeters:
- Turbidity rod: The turbidity can be easily measured in the field with the help of a turbidity rod. It consists of an aluminium rod which is graduated, as to give the turbidity directly in si li ca units (mg/lit)
- Turbidimeter: The turbidity can be measured in the laboratory with the help of instruments called turbidimeter. In general, a turbidimeter works on the principle of measuring the interference caused by the water sample to the passage of light rays.
- Jackson’s candle turbidimeter: The height of water column will therefore, be more for less turbid water; and vice versa. Longer the light path lower the turbidity. Such a turbidimeter cannot measure turbidities lower than 25 JTU. It can be used for natural source only, and it cannot be used to measure the turbidities of treated supplies, for which Baylis’s turbidimeter or modern nephelometers are used.
- Baylis’s turbidimeters: One of the two glass tubes, is filled with water sample (whose turbidity is to be measured) and the other is filled with standard water solution of known t ur bi di ty. The electric bulb is lighted and the blue colour in both the tubes is observed from the top of the instrument.
- Modern Nephelometer: For low turbidity less than 1 unit
- NTU – Nephelometric Turbidity Units
- FTU – Formazin Turbidity Units
- Ratio turbidimeter – River water has maximum amount of Turbidity.
ENVIRONMENTAL ENGINEERING MADE EASY GATE NOTES PDF: CLICK HERE
Colour:
- The presence of colour in water is not objectionable from health point of view; but may spoil the colour of the clothes being washed.
- The standard unit of colour is that which is produced by one milligram of platinum cobalt dissolved in one litre of distilled water.
- For public supplies, the colour number on cobalt scale should not’ exceed 20, and should be preferably be less than 10.
- Colour determined by an instrument is known as tintometer.
Taste and Odour
- The extent of taste or odour present in a particular sample of water is measured by a term called odour intensity, which is related with the threshold odour or threshold odour number.
- Water to be tested is, therefore, gradually diluted with odour free water, and the mixture at which the detection of odour by human observation is just lost, is determined. The number of times the sample is diluted, represents the threshold odour number.
- For public supplies, the water should generally free from odour, i.e. the threshold number should be 1 and should never exceed 3.
ENVIRONMENTAL ENGINEERING PART-1 STUDY MATERIAL FOR SSC JE PDF CIVILENGGFORALL
DOWNLOAD LINK : CLICK HERE
PASSWORD : CivilEnggForAll
ENVIRONMENTAL ENGINEERING SSC JE STUDY MATERIAL PART-2 : CLICK HERE
OTHER USEFUL BOOKS
- RAJASTHAN STAFF SELECTION BOARD (RSSB) JUNIOR ENGINEER DIPLOMA CIVIL ENGINEERING EXAM 2022 – HINDI & ENGLISH MEDIUM SOLVED PAPER – FREE DOWNLOAD PDF (CivilEnggForAll.com)
- ISRO TECHNICAL ASSISTANT EXAM 2022 – CIVIL ENGINEERING – HINDI & ENGLISH MEDIUM – SOLVED PAPER – FREE DOWNLOAD PDF (CivilEnggForAll.com)
- MADHYA PRADESH PUBLIC SERVICE (MPPSC) COMMISSION – ASSISTANT ENGINEER EXAM – MPPSC AE 2021 CIVIL ENGINEERING – SOLVED PAPER WITH EXPLANATIONS – PDF FREE DOWNLOAD
- BIHAR PUBLIC SERVICE COMMISSION (BPSC) ASSISTANT ENGINEER EXAM – 2022 – CIVIL ENGINEERING – SOLVED PAPER – FREE DOWNLOAD PDF (CivilEnggForAll.com)
- ODISHA PUBLIC SERVICE COMMISSION – OPSC AEE PANCHAYATI RAJ EXAM 2021 – SOLVED PAPER WITH EXPLANATION – FREE DOWNLOAD PDF





















